Edgar Huitema is a Scientist, Brewer & Scientific Consultant at https://extranalytics.com. Subscribe to my free newsletter to get the latest advances in science. Better yet, become a paid subscriber to fully enjoy the benefits The Beerologist has on offer.
In this weeks post, we will talk about the impact of ethanol exposure on the yeast genome. Interestingly and at first glance, the work that I will describe here was not necessarily rooted in a question associated with beer brewing. Instead, the observed link between alcohol consumption and cancer occurrence prompted the authors to investigate the molecular mechanisms. To carry out this work, the group of Professor Kevin Verstrepen, used our friend Saccharomyces cerevisiae.
As you will know, yeast when grown in high ethanol (alcohol) concentrations, adapts and becomes more tolerant. Some of these adaptations are genetic, meaning that changes in the cells DNA, result in improved performance. One can ask: Is the mutation rate in yeast always the same, or are there any triggers that would lead to a change in pace?
The answer is "yes".
Previously, and mirroring results found by others, the group concluded in 2015 that exposure to ethanol increases mutation rates. The next question, of course, then became: how does mutation rate change in yeast? Alternatively, what are the molecular mechanisms?
To investigate this problem, these scientists devised a smart trick. S. cerevisiae is susceptible to canavanine (an arginine analogue) through the CAN gene. Disruption of the CAN gene makes yeast resistant to this compound. Therefore, you can use canavanine as a selective agent for the absence of a functional CAN gene. This assay allows for a straightforward experiment.

Grow yeast in the presence or absence of ethanol
Plate the resulting cultures onto plates with or without canavanine.
Count colonies on each and calculate the proportion of resistant cells
Compare proportions between treatments (with and without ethanol)
This experiment allowed the authors to establish that in the presence of 6% alcohol, there is a higher mutation rate in yeast (2.6-3.9x depending on the strain used). In other words, there was a 2.6 to 3.9 fold increase in the number of canavanine resistant mutants in ethanol exposed cultures, when compared to their non-treated counterparts.
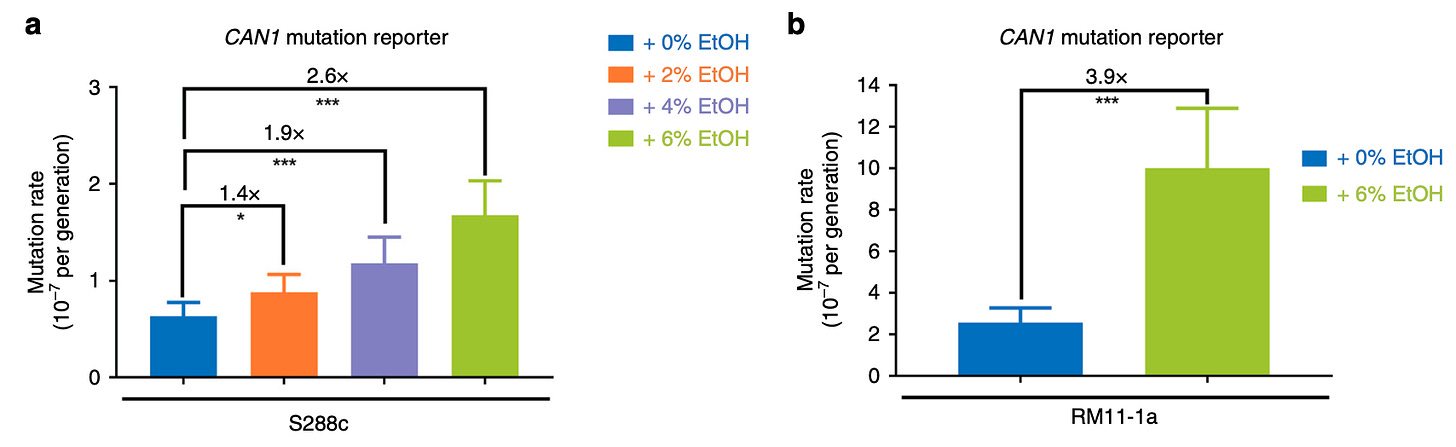
What is the mechanism of ethanol-induced mutation? To investigate this, the authors sequenced the disrupted CAN gene in canavanine resistant strains and asked what sort of modifications occur more frequently in the presence of alcohol. These analyses revealed that C to T substitutions was more frequent. There are several possible mechanisms, leading to C to T substitutions and the authors spent a great deal of time nailing this down.
Gene expression analyses allowed the authors to narrow down the list of possibilities to two mechanisms.
Replication stress. As yeast cells grow, they must replicate (copy) their DNA. Yeast (like almost all other organisms) employs highly regulated pieces of machinery to copy and assemble its chromosomes. These cells use checkpoints to measure progress and when stressed, signal problems. Induction of RNR3 (amongst others) is such a signal. Ethanol exposure increased RNR3 expression, indicating DNA replication stress.
Ethanol exposure also induced gene expression associated with proteotoxic stress. Proteotoxic stress takes place when proteins, produced in the cell, unfold or do not assemble correctly. Ethanol appears to impact yeast proteins' overall integrity, which may include factors that are part of the DNA replication machinery.
The team confirmed both observations with additional experiments I won't discuss here. I will, however, discuss one more discovery that they made.
Replication stress occurs when the DNA copy machine is stalling. A large DNA copier slides along the chromosome reads the sequence and synthesizes the new DNA strand. A particular enzyme (called a DNA-polymerase) is responsible for synthesizing this new DNA strand and correcting its own copy errors. This is how mutations are prevented from happening.
However, the authors found that a stressed replication machine tends to recruit other versions of the polymerase that is less capable of error-repair. This means that under replication stress (induced by ethanol) the copy machine becomes less accurate and prone to errors. These findings help explain the observed acceleration of mutation in the presence of alcohol.
I hope this post is clear and sheds some light on how yeast may adapt when stressed. Next week, I will focus on a topic that is more directly relevant to brewing. Stay tuned!
Cheers,
Edgar, The Beerologist.
Edgar Huitema is a Scientist, Brewer & Scientific Consultant at https://extranalytics.com. Subscribe to my free newsletter to get the latest advances in science. Contact The Beerologist at ExtrAnalytics if you wish to discuss your needs and our research.



